WIR SIND DER QUALITÄT VERPFLICHTET
Akkreditiert nach:
- GMP-zertifiziert durch Swissmedic
- FDA eingerichtet (Kennung der Einrichtung 3008262422)
- ISO/IEC 17025 akkreditiert (STS 0023) durch die Schweizerische Akkreditierungsstelle
ISO/IEC 17025 akkreditiert (STS 0023) durch die Schweizerische Akkreditierungsstelle
Die Schweizerische Akkreditierungsstelle SAS prüft und akkreditiert die Konformitätsbewertungsstellen (KBS) auf der Grundlage der einschlägigen internationalen Normen. Gestützt auf die Akkreditierungs- und Bezeichnungsverordnung vom 17. Juni 1996 und auf Anraten der Eidgenössischen Akkreditierungskommission erteilt die Schweizerische Akkreditierungsstelle (SAS) der Suisse Technology Partners AG die Akkreditierung als Prüfstelle für chemische, physikalische und mechanische Prüfungen von metallischen Werkstoffen, Umweltproben und Elementen in organischen Materialien und Salzen.

GMP-zertifiziert durch Swissmedic
Suisse TP ist von Swissmedic, dem Schweizerischen Heilmittelinstitut, ordnungsgemäss zur Herstellung von Arzneimitteln zugelassen. Diese Bewilligung erlaubt Suisse TP die Durchführung von pharmazeutischen Analysen und die Herstellung von pharmazeutischen Wirkstoffen (API) zur Verwendung in klinischen Versuchen im Rahmen der Guten Herstellungspraxis (GMP). Das Zertifikat basiert auf Inspektionen, die in Übereinstimmung mit den Anforderungen der guten Herstellungspraxis und der Qualitätskontrolle der Pharmaceutical Inspection Convention/Cooperation Scheme (PIC/S) und den Richtlinien der Europäischen Kommission durchgeführt wurden.

FDA established
Die US Food and Drug Administration (FDA) hat die Suisse Technology Partners AG erfolgreich inspiziert. Demnach ist die Suisse Technology Partners AG von der FDA als Prüfstelle für die Analyse von pharmazeutischen Produkten anerkannt und registriert worden. Die Registrierungsnummer für die Suisse Technology Partners AG lautet FEI 300 826 2422.
Darüber hinaus hat die Suisse Technology Partners AG die erstmalige Selbstidentifikation gemäss den Generic Drug User Fee Amendments (GDUFA) erfolgreich bestanden und ist von der FDA akzeptiert worden. Suisse Technology Partners AG verpflichtet sich, diesen Status aufrechtzuerhalten und der FDA die geforderten Identifikationsinformationen jährlich zu bestätigen.

Umgang mit kontrollierten Substanzen - SwissMedic
Suisse Technology Partners verfügt über die eidgenössische Bewilligung zum Umgang mit kontrollierten Substanzen nach Art. 2 Bst. h BetmKV.
Diese Verordnung regelt die Bewilligung und Kontrolle von Betäubungsmitteln, psychotropen Stoffen, Vorprodukten und Hilfschemikalien nach Artikel 2 BetmG und von Rohstoffen und Produkten mit betäubungsmittelähnlicher Wirkung nach Artikel 7 BetmG.

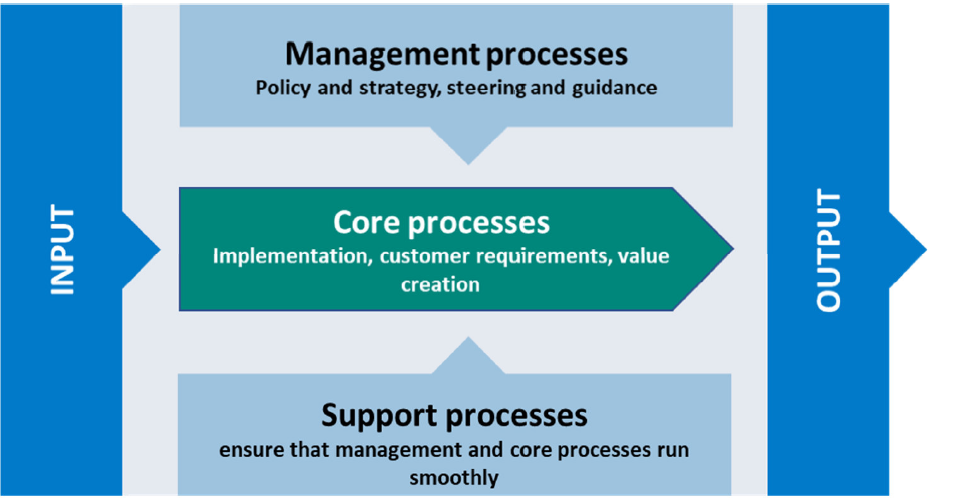
ZENTRALISIERTE VERWALTUNG - EFFIZIENZ UND EFFEKTIVITÄT
Unser zertifiziertes, kunden- und prozessorientiertes Integriertes Managementsystem (IMS) vereint relevante Managementbereiche wie Qualitätsmanagement, Risikomanagement, Datenschutzmanagement, Wissens- und Ideenmanagement, EHS (Environment, Health, Safety) Management in einem einheitlichen Managementkonzept.
INTERNE UND EXTERNE ÜBERWACHUNG
Streng durchgeführte interne und externe Auditverfahren sind ein Muss. Interne Audits untersuchen und beseitigen Probleme im Zusammenhang mit unseren Verfahren und Prozessen.Unsere externe Rechnungsprüfung trägt nicht nur dazu bei, dass die Organisation die geltenden Gesetze, Vorschriften und Normen einhält, sondern bietet auch unabhängige Prüfungen und Analysen unserer internen Kontrollen.Suisse TP ist von vielen Kunden erfolgreich geprüft und qualifiziert worden.










